Highlights
Ultracold molecules poised to compute and simulate
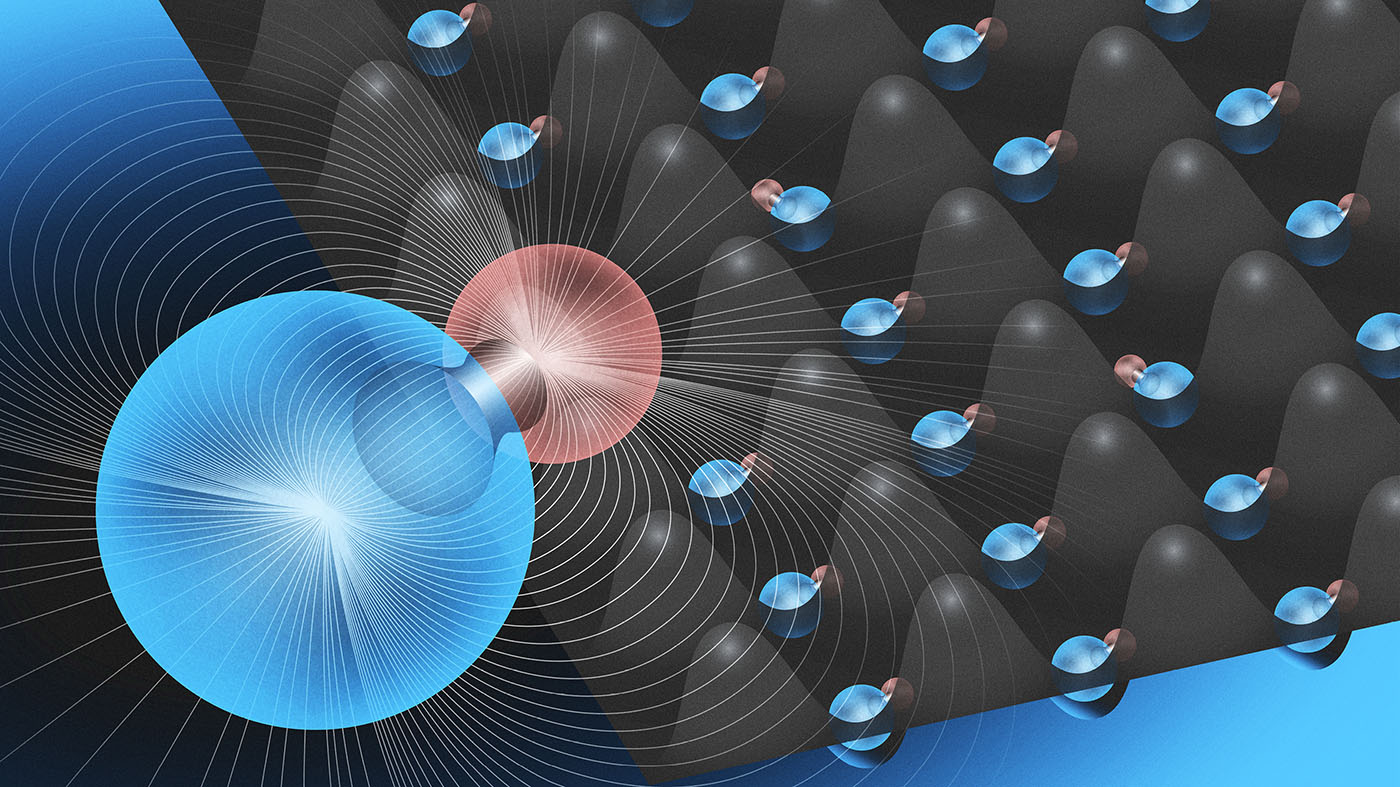 The team makes ultracold molecules by paring a lithium atom with a potassium atom. Each molecule has a large dipole moment, enabling interactions between the molecules. The interactions can bridge long distances in an array which makes it possible to simulate other phenomena in physics.
The team makes ultracold molecules by paring a lithium atom with a potassium atom. Each molecule has a large dipole moment, enabling interactions between the molecules. The interactions can bridge long distances in an array which makes it possible to simulate other phenomena in physics.
A CQT team has pioneered a technique to make ultracold molecules with unprecedented efficiency — and their next step could be turning the molecules into qubits or simulating the physics of phenomena such as a new form of localisation.
In work published in Physical Review Letters on 11 June, CQT Principal Investigator Kai Dieckmann and his team describe their method to move molecules of lithium (Li) and potassium (K) into a zen-like state of zero movement.
“The current result is the outcome of many years of effort. We found our own unique, very original pathway to transition the molecules via an excited state into the ground state,” says Kai. To achieve the scheme, the researchers also needed to design and build a low-phase-noise laser system that could address the necessary molecular states.
With that system in place, the team managed an average 96% transfer efficiency of the 6Li40K molecules from their initial excited state to their ground state. This exceeds efficiencies typically reported for other setups making ultracold molecules with different atoms, which reach up to about 80%.
Kai’s coauthors on the paper are PhD students He Canming, Nie Xiaoyu, and Victor Avalos, CQT Research Fellow Yang Anbang and group alumni Sofia Botsi and Sunil Kumar.
Laser precision
The team make thousands of molecules many times each day in their lab by cooling clouds of lithium and potassium atoms together. The atoms easily bind in pairs, but the molecules are born in excited states that are vibrating violently. The researchers need to quell the molecules’ vibrations, moving these molecules into their ground state to do further experiments.
A high efficiency transfer is good because it leaves more molecules for the next steps. Any molecules still in their excited state remain as undesirable impurities.
In a 2020 paper in Physical Review Letters, the researchers identified a set of energy levels they could use to move their molecules to the ground state by a process called Stimulated Raman Adiabatic Passage (STIRAP). “In atoms, often STIRAP is quite easy. In molecules, it’s much more challenging technologically and the physics is more involved,” says Kai.
STIRAP involves boosting the atom or molecule up through an excited state on the way to the ground state. Each transition is driven by a different frequency of light. For atoms, the light from a single laser can usually be manipulated to drive both transitions.
One challenge for the molecules was that it needed two different lasers, because the frequencies were too different to make from one. Another challenge was that the team was pushing the LiK molecule through an excited state that turned out to have a broad linewidth, meaning they needed to use more laser power for longer.
At first, they could not make it work. “There was a moment when we were a bit desperate and don't know what was going wrong, so we started analysing, something like a checklist. We looked at the literature and found out there could be a problem with the laser noise,” says co-author and CQT Research Fellow Yang Anbang.
To reduce the laser noise, the team found a simple fix. Standard diode lasers are usually very short, amplifying light inside a reflective cavity about 2cm long. The researchers rebuilt the diode lasers to have a bigger cavity about 20cm long, which stabilises the output.
“The students were really hanging in there, working very hard and overcoming those problems in a series of papers. Now we have finally this chapter closed,” says Kai.
Doing more with molecules
The researchers measured the lifetime of their ground-state molecules to be around 5 milliseconds, likely limited by ultracold chemical reactions. They also confirmed that LiK has a large permanent dipole moment. This dipole moment is a selling point for future work because it enables long-range interactions between the molecules.
For example, the team is interested to investigate the impact of interactions on the localisation of excitations in a lattice of ultracold molecules. The interactions could also be exploited for performing gates on qubits. The rotational states of ultracold molecules are thought to be promising candidates to store qubits or even qudits, in which information is encoded in a base beyond binary. The team plan to measure the suitability of their molecules as qubits too.
For the next step for both future directions, the team has loaded the molecules into optical lattices — formed of criss-crossing laser light that organises the molecules into a regular grid pattern of lattice sites — to address them individually. In the lattices, chemical reactions are suppressed and the molecule’s lifetime is long.
With one molecule per lattice site, the team will be able to precisely control the state of each individual molecule — that means their motional, vibrational and electronic states. “Our quantum control can extend to every degree of freedom. The possibilities for our setup now are very exciting,” says Kai.
 Some of the molecule-making team in the lab. Pictured from left to right are Anbang Yang, Victor Avalos, He Canming and Nie Xiaoyu.
Some of the molecule-making team in the lab. Pictured from left to right are Anbang Yang, Victor Avalos, He Canming and Nie Xiaoyu.